What makes a vaccine?
Vaccines are miracles of modern medicine that have saved hundreds of millions of lives. But behind every vaccine are years of careful research and rigorous testing. Here’s what you need to know.
What is a vaccine?
Vaccines work by harnessing our bodies’ natural defenses. Vaccines contain an antigen, or a substance that prompts the body to produce antibodies. Antigens can be a weakened form of a virus or bacteria or related pieces of genetic material. Contact with the antigen mimics an infection without fully causing one, prompting the immune system to create antibodies and effectively training it to fight off that particular virus or bacteria in the event of future exposure.
A (very) brief history of vaccine development
The first vaccine was developed by Edward Jenner, a physician in 18th century England. Jenner noticed farmers who had contracted cowpox, a mild viral infection that causes discomfort but few serious symptoms in either cows or people, appeared not to catch smallpox. After years of observations and experiments, Jenner verified that immunity brought about by exposure to cowpox also conferred protection against the related but far deadlier smallpox virus.
Jenner had identified the basic principles that have made countless subsequent vaccines possible, but vaccine development has come a long way in the centuries since.
Here’s how it’s done now.
Step 1: Selecting an antigen
The first step in creating a new vaccine is identifying the antigen, which has to be recognized by the body as a threat so that it prompts a protective immune response, but without causing infection.
In some cases, such as measles vaccines, the antigen is a weakened form of the virus that triggers an immune response without causing severe illness or health risks. In others, such as COVID-19 vaccines, the antigen is a component protein which similarly triggers the body’s defenses but does not cause infection.
Identifying the correct antigen is not as simple as it sounds. In fact, it often takes years of research, often conducted at universities or by private industry, to identify antigens to include in a new vaccine.
Step 2: Pre-clinical research
Once the antigen is selected for a candidate vaccine, it moves into pre-clinical testing. During the pre-clinical phase, researchers use human cell cultures or animal subjects to test the safety and efficacy of a vaccine before it can be tested in humans.
Pre-clinical testing is rigorous and extensive. It needs to provide evidence on the safety profile of a vaccine, the strength and efficacy of the immune response it prompts, the correct dosage amount and frequency, and more.

Only once these tests have been conducted and thoroughly analyzed can the vaccine development process move forward. Researchers compile all the results of their lab research and pre-clinical testing, along with any relevant information about production, and submit it for review by the Food and Drug Administration (FDA). Once the FDA has verified that all research followed scientific best practices and demonstrates the vaccine candidate is safe and likely to be effective, it can move on to clinical trials.
Step 3: Clinical trials
Clinical trials—the stage of vaccine development where the vaccine is first tested in humans—take place in three phases.
Phase 1 involves small groups of 20 to 100 people who receive the vaccine and are monitored for side effects. While pre-clinical research must provide strong evidence of a vaccine’s safety and efficacy, phase 1 offers a critical first look at how exactly a human immune system—not an animal or culture proxy—responds to the antigen.
Phase 2 expands the trial to include several hundred participants. In addition to testing safety and efficacy at a progressively larger scale, phase 2 trials often select participants based on their age, health, and other characteristics to ensure the vaccine will work in its intended population. For example, a shingles vaccine would be tested primarily in older adults as they are the main population affected by shingles. Phase 2 trials also aim to enroll participants from a variety of backgrounds so that safety and efficacy data is representative as possible.
However, it’s worth noting that childhood vaccines typically do not jump straight from small phase 1 trials to trials in children. Instead, childhood vaccines use a “step-down” approach, meaning vaccines are first trialed in adults, before being trialed in teens, then children, then babies (if applicable).
Phase 3 involves thousands of participants to generate extremely extensive data on the new vaccine. The much larger sample size makes it easier to identify very rare side effects and allows researchers to compare vaccinated participants with those given a placebo, providing more concrete evidence into just how likely the vaccine is to reduce disease. The scale of phase 3 studies also allows researchers to refine the manufacturing process for the new vaccine and prepare for commercial-scale production, if and when approval is granted.
Each phase may take several years to allow for rigorous testing and analysis, but in general, there is no predetermined timeline for development, and the precise amount of time typically depends on how well understood the pathogen and disease targeted by the candidate vaccine are. In rare public health emergencies, like COVID-19 or mpox, when a vaccine is urgently needed, phases may overlap or be expedited to ensure rapid approval, but not at the expense of standards of evidence and safety.
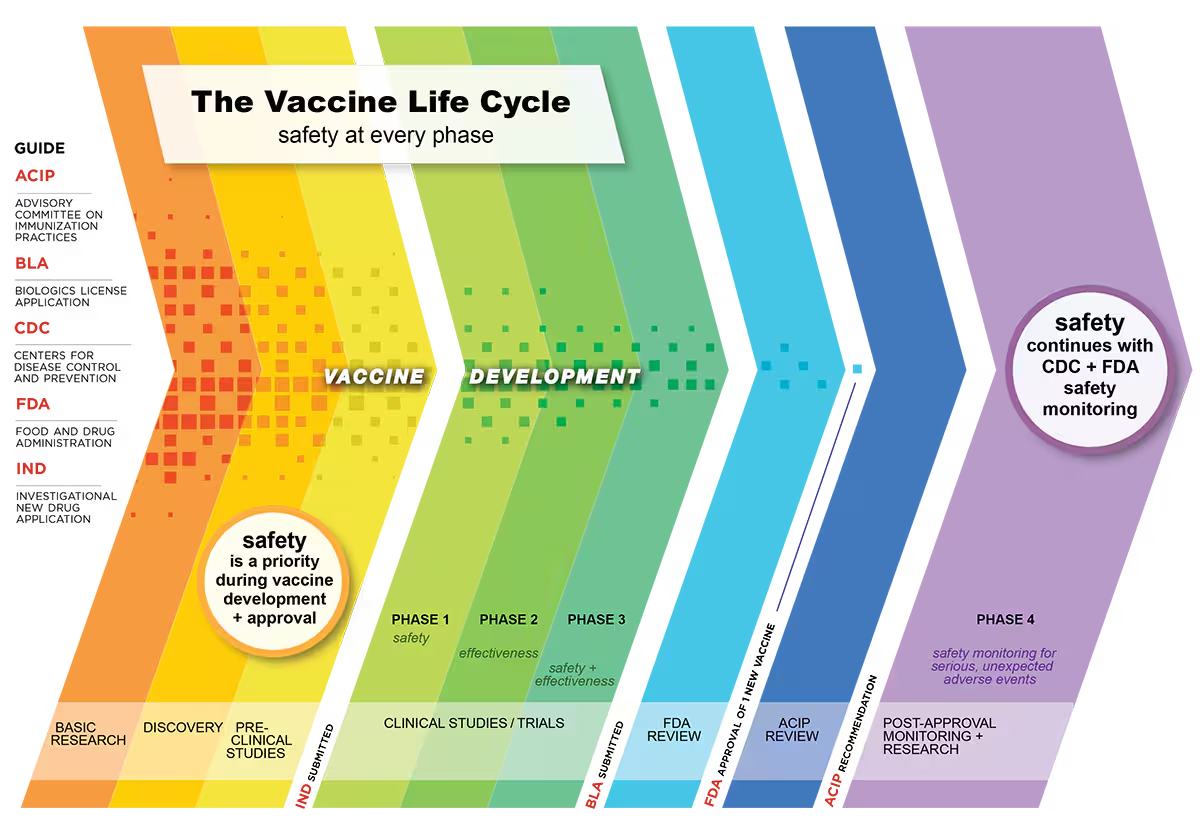
Step 4: Approval and licensure
Once clinical trial data from all three phases has been collected and analyzed, the company submits a Biologics License Application (BLA) to the FDA, which will allow them to distribute and market the new vaccine in the U.S.
BLAs are reviewed by teams of FDA experts including physicians, chemists, statisticians, microbiologists, and pharmacologists, who together analyze the totality of evidence for the vaccine. Other factors, such as the severity of the disease being targeted for prevention and the availability of other treatments, may also be considered.
Even once a vaccine is approved, the FDA still requires ongoing surveillance to identify any highly uncommon adverse side effects or longer-term complications not detectable during the clinical trial duration. Sometimes, the FDA may even require additional studies (called phase 4 trials) to further assess potential risks.
In other words, at every stage of the vaccine development process, there are safeguards and safety reviews in place to catch possible risks to recipients.
Conclusion
Vaccine development has come a long way since Dr. Jenner first theorized that cowpox provided immunity against smallpox. It is now a highly structured, extremely rigorous, and thoroughly accountable process with multiple steps of research, review, and analysis to ensure that any vaccine to reach the market will be as safe and effective as possible.
But the development of a new vaccine isn’t the end of the story—it’s just the beginning. When the scientists’ work of research, development, and testing ends, our work—the work of ensuring everyone has access to these modern-day miracles—begins.

