Stopping the Silent Pandemic
From a High-Level Meeting at this year’s UN General Assembly to this week’s international observance, anti-microbial resistance is increasingly a major focus in global health. Here’s what you need to know.

What is anti-microbial resistance?
Anti-microbial resistance (AMR) is a process through which bacteria, viruses, fungi, and parasites develop resistance to medicines designed to kill them. When these germs are no longer treatable with antibiotics and other antimicrobials, the infections they cause become harder—in some cases, impossible—to treat.
AMR is an adaptive process. When antibiotics are used to treat an infection, they kill most of the harmful microbes that are causing it. But the microbes that survive continue to multiply, passing on their genetic resistance. Over time, these microbes can even share their antimicrobial-resistant genetic traits with other, unrelated microbes through direct physical contact in a process called horizontal gene transfer. That means the development of durable antimicrobial resistance by any one harmful microbe risks making a whole host other germs harder to treat with antimicrobial drugs.
The development of AMR is a natural process, but it is seriously exacerbated by the overuse or misuse of antibiotics and other antimicrobial medicines in both humans and animals. According to the CDC, about one-third of antibiotic prescriptions in the United States are unnecessary. Most commonly, antibiotics are incorrectly prescribed for respiratory symptoms caused by viruses that do not respond to them, like sinusitis, bronchitis, or the common cold. In lower-income countries, where diagnostics, quality healthcare, and regulation of antibiotic use are all more limited, rates of overuse are likely even higher.
The routine use of antibiotics in agriculture, when they are often used in healthy animals to promote growth or proactively prevent infections, also contributes to the development and spread of AMR. In some countries, livestock already account for as much as 80% of total antibiotic use. And at the global level, the share of antimicrobials used to treat animals is expected to grow, which could further accelerate AMR’s spread.
The silent pandemic
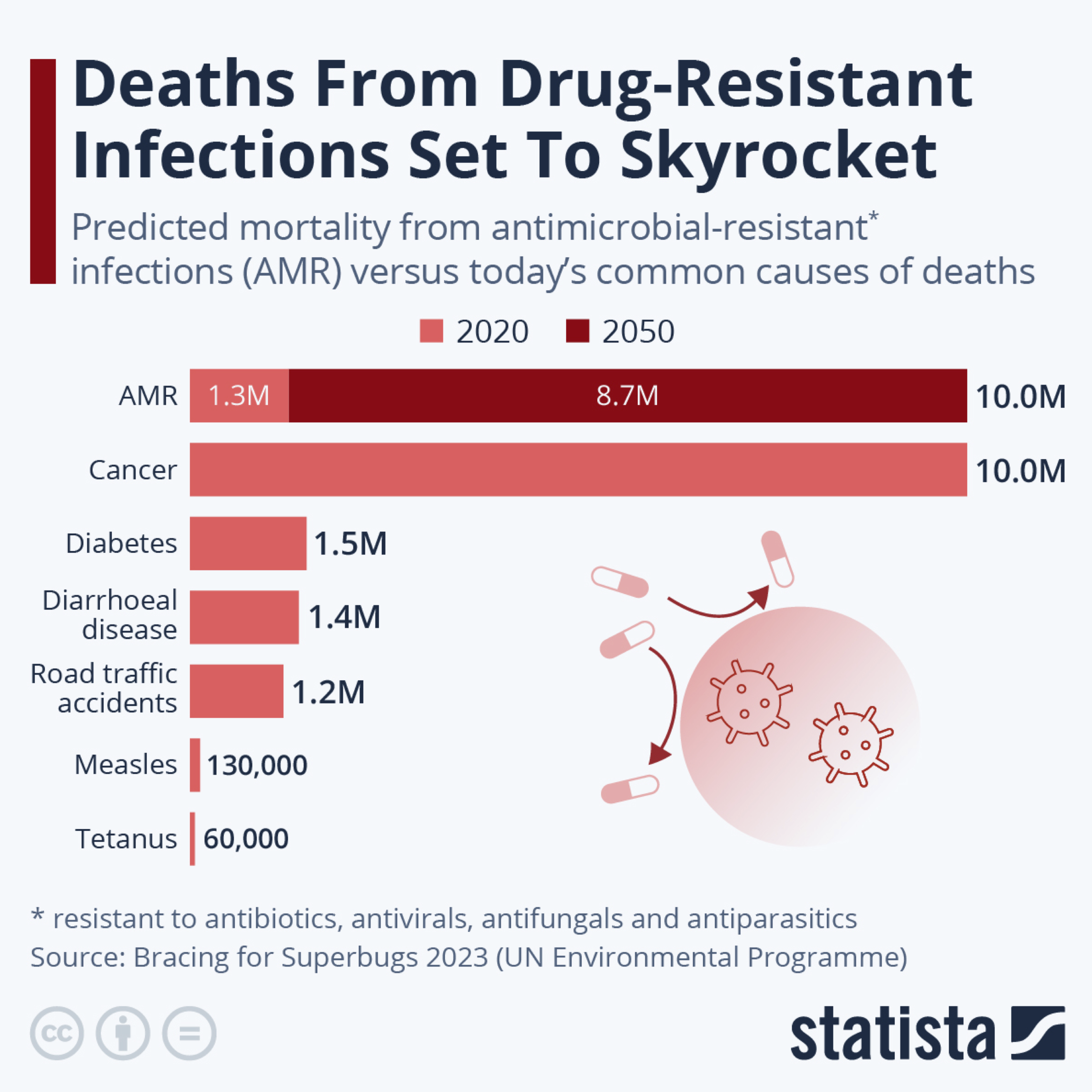
Discussions of AMR often focus on slowing its development and mitigating its spread, but it’s already a significant public health problem with deadly consequences. Every year, AMR directly causes more than one million deaths—more than HIV/AIDS or malaria—and contributes to more than five million. Of the five million annual deaths attributable to AMR, about one million are children younger than five. This massive but under-discussed death toll has led scientists to dub AMR the “silent pandemic.”
AMR is a threat everywhere. In the United States, there are nearly three million antimicrobial-resistant infections every year, causing about 35,000 deaths. But deaths from AMR tend to be concentrated in lower-income countries, where denser living conditions, lack of access to clean water and sanitation, and more regular contact with livestock and other animals all contribute to the transmission of germs. Lack of access to healthcare, in particular to second- or third-line treatment options once an infection has proven resistant to basic antibiotics, is also a factor.
According to current estimates, AMR is likely to cause or contribute to some 10 million annual deaths by 2050.
Slowing the spread
While we cannot prevent AMR entirely, we have the ability to greatly mitigate its impact. On the individual level, we can all avoid misusing antibiotics by making sure to take them only when prescribed by a doctor for a bacterial infection. And we can all practice good hygiene to prevent the spread of germs and avoid infections that will require antibiotic use. The less we as a society use antibiotics overall, the more we slow the spread of AMR.
But one of the most important ways to limit the spread of AMR is a familiar solution: vaccination.
There are a number of ways vaccines can help us fight back against AMR. Vaccines that prevent bacterial infections, like the pneumococcal vaccine, can directly reduce the number of infections that require treatment with antibiotics. Most vaccines, by preventing serious illness, can also help prevent the development of secondary complications that may require antibiotic treatment, like a case of pneumonia that develops in a patient whose immune system has been weakened by the flu. Finally, simply by keeping people healthy and limiting the number of serious illnesses, doctor’s visits, and hospital admissions, vaccination reduces the risk of inappropriate prescription of antibiotics.
The expansion of vaccine access has already made a real difference in protecting children from drug-resistant infections. While AMR-related deaths have increased slightly over the past 30 years, AMR-related deaths in children under five have decreased by more than 50% over the same period, thanks in large part to pneumococcal vaccination and the decline in cases of drug-resistant pneumonia in children.
According to the WHO, simply expanding access to currently licensed vaccines could prevent more than 100,000 deaths attributable to AMR every year. The same study estimates that current vaccines could also save $861 million in hospital costs and $5.9 billion in productivity losses due to vaccine-preventable infections every year. Vaccines currently in development, including improved tuberculosis vaccines, could reduce the human and economic cost of AMR even further.
Vaccines are not a silver bullet. Improved agricultural practices, better diagnostics, and access to clean water and sanitation to limit infections and slow the transfer of resistant genetic traits between pathogens are all equally important to containing AMR. But simply expanding access to the vaccines we already have has the potential to save tens of thousands of lives and significantly slow AMR’s spread.
Political commitments
AMR, and reducing its spread, has increasingly been on the international agenda. In September, the 79th UN General Assembly featured a High-Level Political Meeting on Anti-Microbial Resistance, a rare spotlight for an often under-discussed issue.
The subsequent Political Declaration adopted by Member States commits to reducing deaths from AMR by 10% by 2030 and requires that all countries develop and implement national action plans to address AMR by 2030. The Declaration also embraces a multidisciplinary approach to the issue by formalizing the cooperative role of the WHO, the Food and Agriculture Organization, the World Organisation for Animal Health, and the UN Environment Program. The recognition that AMR requires a “One Health” approach—which accounts for the interconnectedness of human, animal, and planetary health—will be critical to addressing its spread.
And just this weekend, representatives of the health, environmental, and agricultural ministries of 57 countries met in Saudi Arabia to further define the way forward, including a new independent panel to collect evidence on AMR and a call for further research into new tools, including vaccines, to combat it.
Scientific research and policy development take time, but the spotlight on AMR has never been brighter, and the political will to address it has never been more in evidence. As leaders commit to addressing this quiet crisis, it’s up to us to remind them that an investment in health today is protecting a healthier tomorrow for us all.
AMR affects, and can be affected by, virtually all aspects of society in some way. Follow us on Instagram this week and next as Shot@Life’s College Ambassadors explain the fascinating and sometimes-surprising relationships between AMR and the UN’s Sustainable Development Goals.

